Current Oncology | Free Full-Text | Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients

Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events: Methods for item selection in industry-sponsored oncology clinical trials - Peter C Trask, Amylou C Dueck, Elisabeth Piault, Alicyn Campbell, 2018

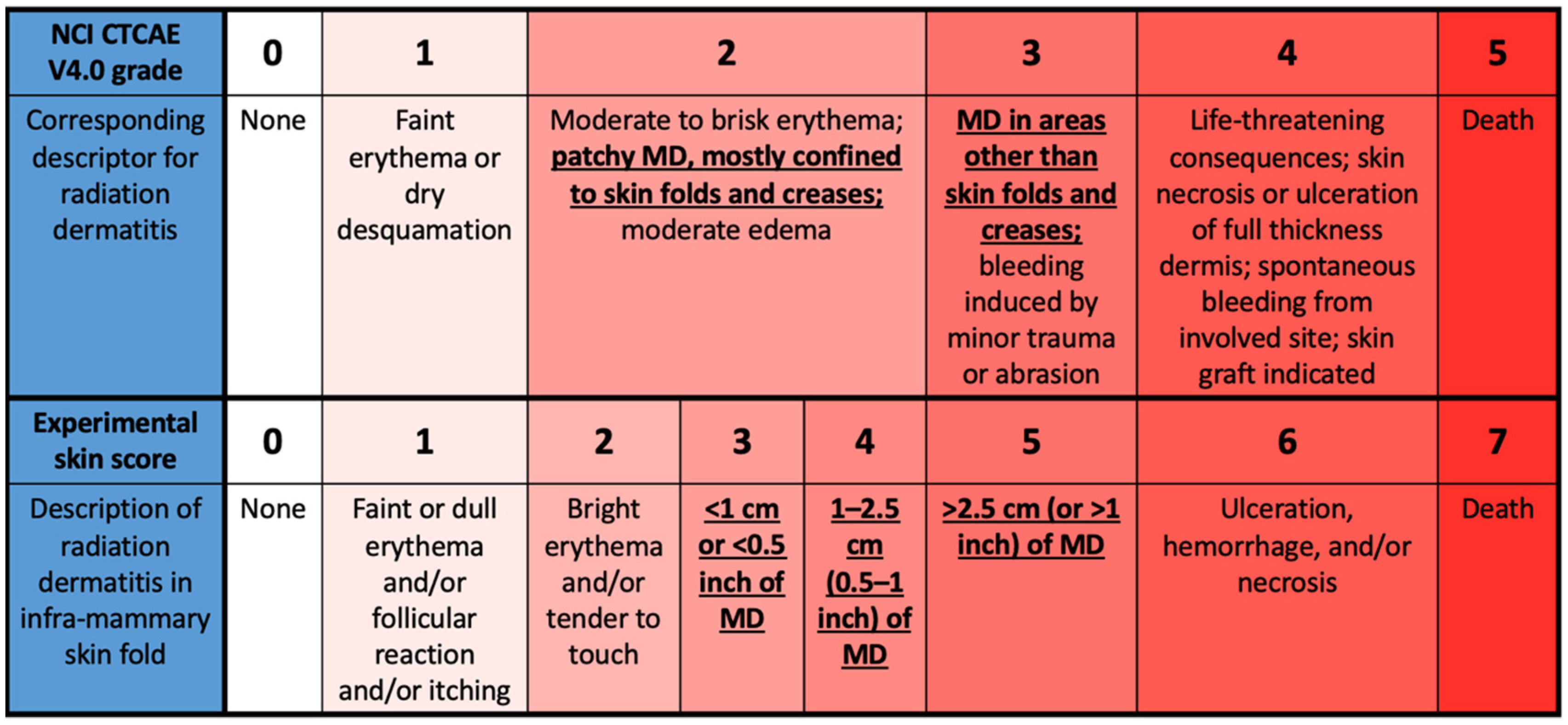
RTOG and CTCAE v4.0 toxicity scales for acute skin reaction and ulceration. | Download Scientific Diagram

Establishing a common metric for patient-reported outcomes in cancer patients: linking patient reported outcomes measurement information system (PROMIS), numerical rating scale, and patient-reported outcomes version of the common terminology criteria ...

Common Terminology Criteria for Adverse Events (CTCAE) for diarrhea and... | Download Scientific Diagram

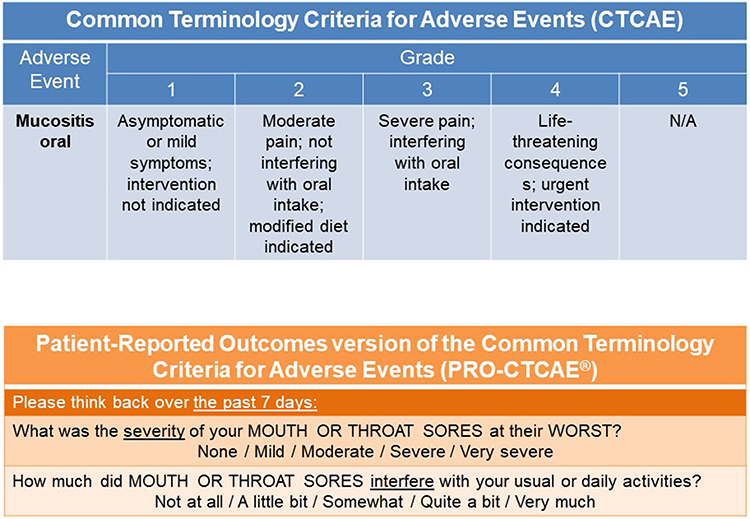
Full article: Clinician and Patient Reporting of Symptomatic Adverse Events in Cancer Clinical Trials: Using CTCAE and PRO-CTCAE® to Provide Two Distinct and Complementary Perspectives

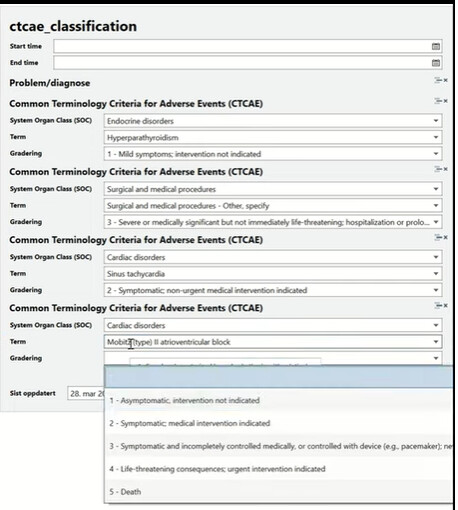
Use and misuse of common terminology criteria for adverse events in cancer clinical trials | BMC Cancer | Full Text

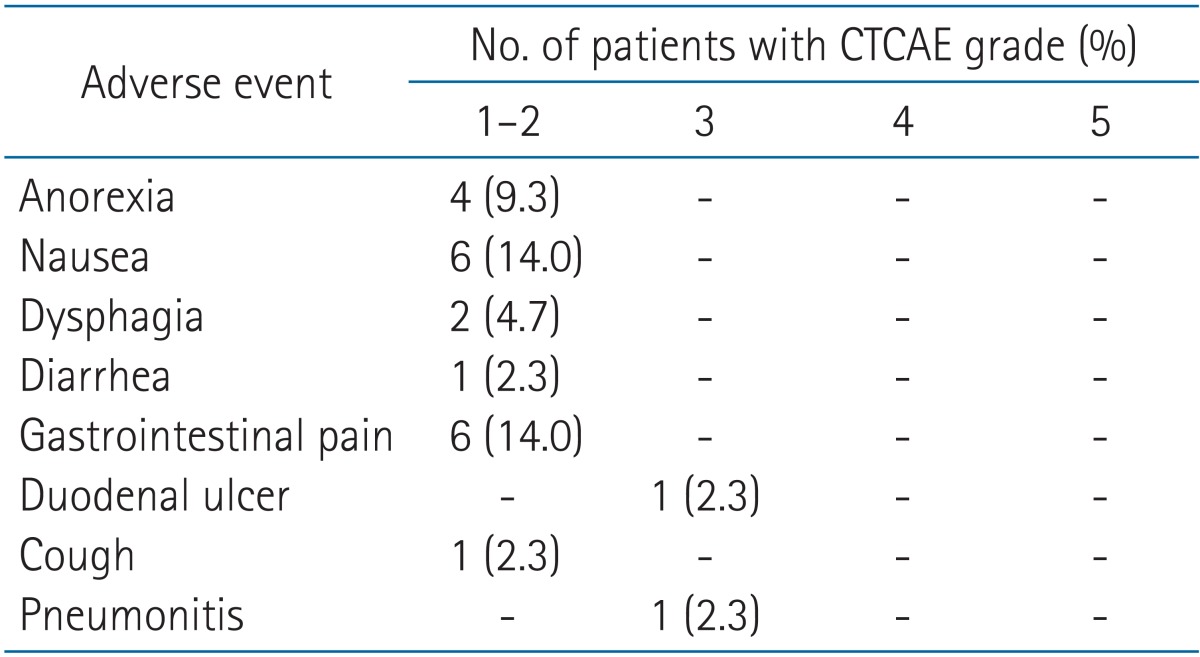
Toxicity grading scale in the common terminology criteria for adverse... | Download Scientific Diagram

Veterinary cooperative oncology group – common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1 - 2016 - Veterinary and Comparative Oncology - Wiley Online Library